Design and Development Process for Medical Device Manufacturing: From Concept to Reality
Introduction
In the fast-evolving world of medical technology, the design and development process for medical devices is a critical process that develops of safe, efficient, and innovative products. For a medical device manufacturing company, this process is the backbone of their success, as it directly impacts the quality and functionality of the devices they produce, as well as patient efficacy and future revenue. In this blog, we will explore the key stages involved in the design and development process for a medical device, highlighting the importance of each step.
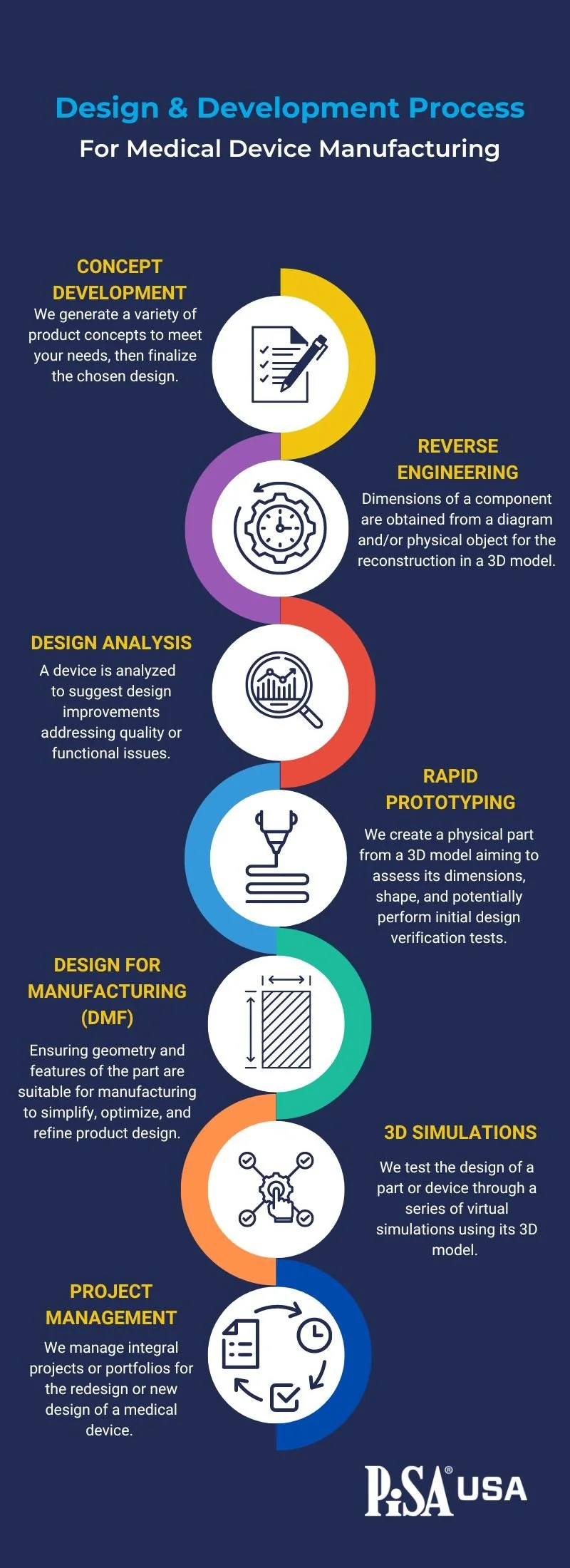
1. Concept Development
The journey of creating a medical device starts with concept development. At this stage, the initial client requirements and specifications are meticulously reviewed. This is a collaborative engagement, where understanding the client's needs and the challenges they want to address is vital in generating potential product concepts. The design team brainstorm and explore various options to meet these requirements. Multiple concepts are developed, considering factors like usability, functionality, safety, and cost-effectiveness.
This iterative process continues until a design stands out as the most viable solution. At this stage the design can be frozen, which marks an essential milestone where the chosen design selected for further development.
2. Reverse Engineering
In some cases, a medical device manufacturing company may be required to improve an existing product or component. Reverse engineering comes into play when there is no 2D diagram or 3D model available. This frequently occurs with older products or where a relationship with a previous design partner has broken down. The process involves obtaining the dimensions of the component from a physical object or a diagram and then reconstructing it in a 3D model. CT Scanning can also aid this process. Reverse engineering helps capture the design intent, which is essential when modifications or improvements need to be made to an existing part without access to the original design documentation.
3. Design Analysis
Design analysis is a crucial step to ensure that the medical device meets the highest standards of quality and functionality. The process involves reviewing an existing device or part and identifying any potential issues or areas for improvement. By conducting thorough design analysis, manufacturers can eliminate quality or functional problems, leading to a safer and more efficient product.
4. Rapid Prototyping
Once a concept is finalized, rapid prototyping comes into play. Rapid prototyping is the process of manufacturing a physical part directly from a 3D model. This step allows designers to visualize and assess the shape, size, and functionality of the component. Different materials can be used during prototyping to evaluate their performance and suitability for the device.
Rapid prototyping also facilitates preliminary tests for design verification, ensuring that the chosen concept aligns with the desired requirements.
5. Design for Manufacturing
Design for Manufacturing (DFM) is a vital process to ensure that the product design is manufacturable and efficient in the production phase. During this stage, the geometry and features of the part are thoroughly examined to optimize and refine the design.
DFM helps identify and address potential manufacturing challenges, reducing production costs, and shortening lead times. A well-designed medical device that considers manufacturing constraints leads to improved production efficiency and ultimately benefits the end-users.
6. 3D Simulation
The 3D simulation process allows designers to virtually test the functionality and performance of a part or device before moving to physical prototyping. Through a series of virtual simulations using the 3D model, the team can analyze various scenarios and make necessary adjustments.
By simulating real-world conditions and potential stress points, designers can validate the design's integrity and optimize its performance before the costly phase of physical production.
7. Project Management
Throughout the design and development process, effective project management is essential for a successful outcome. Managing projects for medical device design requires meticulous planning, resource allocation, and adherence to timelines. It requires the Project manager to hold both teams accountable for deliverables.
Project management ensures that all stages of the process, from initial requirements research to design validation, are well-coordinated and executed. It may involve collaboration with international design firms or utilizing internal resources to achieve a functional design that meets all established requirements.
Conclusion
The design and development process for a medical device manufacturing company is a complex yet essential journey that involves creativity, analysis, and precision. From concept development to project management, each stage plays a crucial role in ensuring the creation of safe, innovative, and efficient medical devices. By integrating cutting-edge technology, iterative design practices, and a keen focus on client needs, medical device manufacturers can continue to push the boundaries of medical technology and improve patient outcomes.